Abstract
Introduction
After therapy or stem cell transplantation, multiple myeloma patients achieving complete response (CR) or stringent complete response (sCR) can still have a significant risk of disease relapse, illustrating the importance of using highly sensitive methods for minimal residual disease (MRD) detection and prognostication. Two techniques used clinically for MRD detection include multiparametric flow cytometry (FC), which has a sensitivity down to 2-6 X 10-6 of cells, and next-generation sequencing (NGS)-based assay for detection of patient-specific clonal IGH VDJ gene sequences associated with the neoplastic plasma cells (PC). We determined the clonal characterization success rate of plasma cell neoplasm samples from a single institution in a clinical lab, using a commercially available NGS-based assay, Lymphotrack® (Invivoscribe, San Diego, CA). The characterized clonal sequences were used for MRD detection in subsequent monitoring samples, and the results were compared to concurrent FC findings.
Methods
DNA was extracted from fresh marrow or formalin-fixed paraffin-embedded (FFPE) tissue, and amplified by PCR reactions using primers sets for IGH Leader, FR1, FR2, FR3 regions, and IGK. Sequencing was performed on the Illumina MiSeqTM Platform, and sequence analyses were performed using the Lymphotrack® software, and MSK-Lymphoclone, a software developed at our institution. Disease-associated clonal sequences were characterized based on predefined clonal calling criteria and stored. In subsequent samples sent for disease monitoring, a search for sequencing reads with high homology (>99%) to the patient-specific sequences was performed for MRD detection. 10-color FC for PC analyses were also performed on the same samples at our institution (Roshal M, et al. Blood Adv 2017;1(12):728-32), with a target minimum of 3 million cells for MRD analyses.
Results
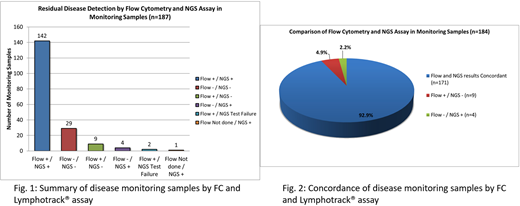
Overall, clonal characterization was successful in 235/251 cases (93.6%), with no difference in number of sequencing reads between the successful and unsuccessful cases (p=0.24). Higher success rate was observed among cases with higher aspirate PC counts: ≥5% (95.6% success rate) and ≥10% (98.1% success rate). IGH FR1 and Leader primers together characterized 214/251 cases (85.3%), while the remaining cases required additional primers. The characterized clones showed high median somatic hypermutation (SHM) rate of 8.1% (range: 0.0-29.0%), as well as IGH V and J segment usage bias: V3 (50.2%), followed by V4 (20.3%); J4 (43.4%), followed by J6 (27.5%), concordant with prior literature. 187 samples from 124 unique patients were tested by the Lymphotrack® assay for monitoring purposes, of which the diagnostic clones were detected in 147/187 samples (78.6%), with no difference in number of sequencing reads between cases with and without detectable clone (p=0.35). Within the short median time interval of 9.5 months between the characterization and monitoring samples, most clonal sequences remained stable. In 2 cases, new clonal sequences emerged in subsequent samples. Overall, FC and Lymphotrack® showed high concordance rate for MRD detection (92.9%) (See figures). All discordant cases showed <5% PC by aspirate differential counts and CD138 immunostains. FC+/NGS- cases (9/184, 4.9%) showed abnormal PC comprising a median of 0.00095% of WBC by FC, while FC-/NGS+ cases (4/184, 2.2%) showed detectable clone at a median of 0.0405% of sequencing reads. Sampling differences might have contributed to the discrepancies. Additionally, in the FC+/NGS- cases, neoplastic subclones might be present at very low level in the characterization samples, below threshold for clonal calling, and therefore could not be specifically tracked in subsequent samples.
Conclusions
Our study demonstrated high clonal characterization success rate for plasma cell neoplasms using the Lymphotrack® assay when multiple primers sets were used, and the assay showed concordance with FC in MRD detection for the majority of cases. MRD detection sensitivity can be limited by low sample concentration/volume. Furthermore, the presence of very low level neoplastic subclones in the characterization samples might hamper clonal calling and detection in subsequent samples.
Ho:Invivoscribe, Inc.: Honoraria. Landgren:Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; Karyopharm: Consultancy. Arcila:Invivoscribe, Inc.: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal